CTMS - Manage Your Trial Portfolio with Confidence
From enrollment through monitoring to closure. Complete audit trail and compliance built-in.

From enrollment through monitoring to closure. Complete audit trail and compliance built-in.

A Clinical Trial Management System (CTMS) is the operational backbone of clinical research organizations. It centralizes all aspects of trial management—from site selection and patient enrollment to monitoring visits and study closeout. Think of it as your mission control for clinical trials: tracking timelines, managing resources, coordinating across sites, and maintaining the documentation trail that regulators expect to see.
Many clinical operations teams still manage trials across disconnected tools: Excel spreadsheets for enrollment tracking, email chains for site communications, separate document repositories, and manual reports for sponsors. This fragmentation creates data silos, increases error risk, and makes real-time visibility impossible. When enrollment is falling behind or a site has compliance issues, you need to know immediately—not when someone manually updates a spreadsheet next week.
We designed our CTMS for clinical operations professionals who need real-time visibility, not just data storage. Our focus: workflow automation, cross-site coordination, and regulatory compliance built into every module.
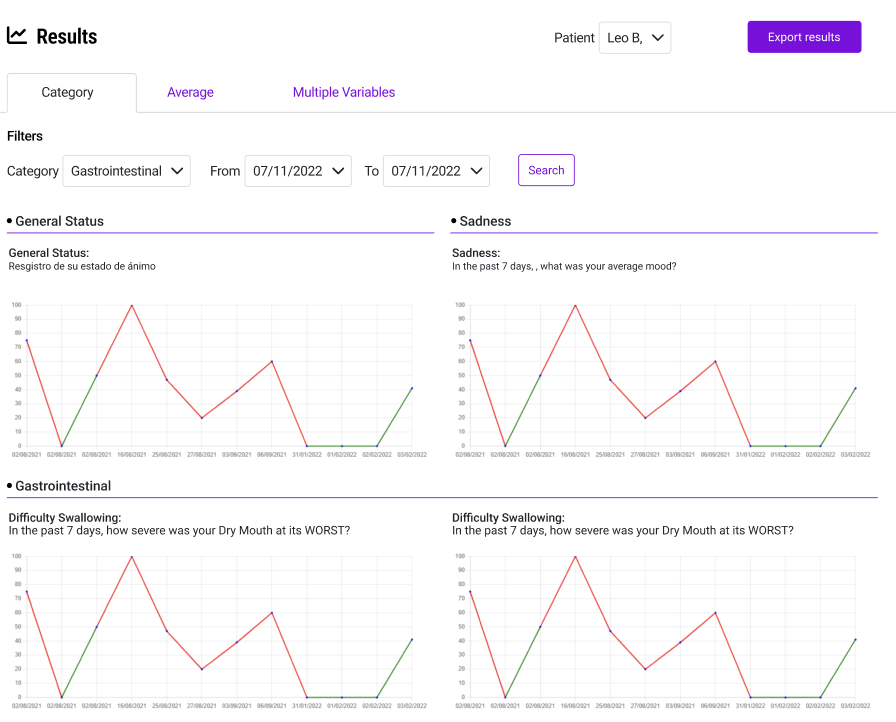
Start your trials on solid ground with comprehensive planning tools. Define protocols, set milestones, allocate resources, and track enrollment progress in real-time across all sites. Our enrollment dashboard shows you exactly where you stand against targets, which sites are underperforming, and where bottlenecks are forming—so you can intervene before delays impact your timeline. Every protocol amendment is version-controlled and traceable, giving you the documentation trail regulators expect.
Start your trials on solid ground with comprehensive planning tools. Define protocols, set milestones, allocate resources, and track enrollment progress in real-time across all sites. Our enrollment dashboard shows you exactly where you stand against targets, which sites are underperforming, and where bottlenecks are forming—so you can intervene before delays impact your timeline.

Keep your trials compliant with integrated monitoring tools. Schedule and track monitoring visits, document findings, manage corrective actions, and maintain issue logs—all in one system. When a monitor identifies a protocol deviation or data discrepancy, it's logged immediately with automatic notifications to the right stakeholders. No more email chains about follow-up actions. Our CTMS ensures monitoring findings are tracked to resolution, with complete audit trails that satisfy regulatory inspectors.
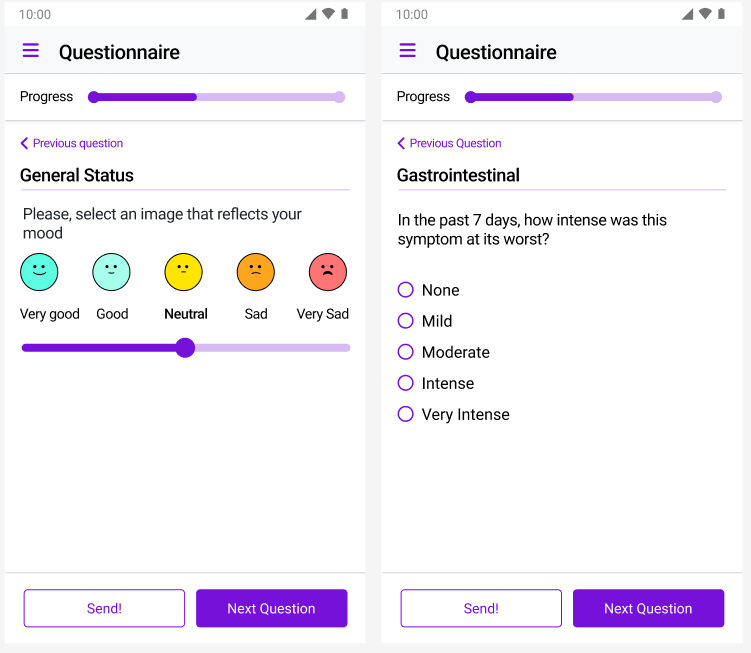
Keep your trials compliant with integrated monitoring tools. Schedule and track monitoring visits, document findings, manage corrective actions, and maintain issue logs—all in one system. When a monitor identifies a protocol deviation or data discrepancy, it's logged immediately with automatic notifications to the right stakeholders.

Make data-driven decisions with comprehensive analytics across your trial portfolio. Track KPIs, visualize trends, and generate sponsor reports in minutes instead of hours. Our reporting tools pull real-time data from all modules—enrollment metrics, site performance, monitoring findings, and budget tracking—giving you portfolio-level visibility that helps you optimize operations and demonstrate value to sponsors. Customizable dashboards mean each stakeholder sees the metrics they care about, without manual report generation.
Make data-driven decisions with comprehensive analytics across your trial portfolio. Track KPIs, visualize trends, and generate sponsor reports in minutes instead of hours. Our reporting tools pull real-time data from all modules—enrollment metrics, site performance, monitoring findings, and budget tracking—giving you portfolio-level visibility.

"Built for clinical operations teams managing multi-site trials, our CTMS brings together planning, monitoring, and reporting in one platform—with CFR 21 Part 11 compliance and complete audit trails built into every workflow."

Let's discuss your clinical trial challenges. Schedule a consultation with our team.
