ePRO - Mobile-First Patient Data You Can Trust
2,400+ surveys completed. Real-time adverse event alerts. Compliance built-in.

2,400+ surveys completed. Real-time adverse event alerts. Compliance built-in.

Electronic Patient Reported Outcomes (ePRO) systems allow patients to report symptoms, side effects, and quality of life data directly through digital devices—without clinical staff intervention. In clinical trials, ePRO captures the patient's voice in real-time, providing researchers with accurate, timestamped data that meets regulatory requirements. Unlike paper diaries or periodic clinic visits, ePRO gives sponsors continuous insight into how patients actually feel between appointments.
Many clinical trials still rely on paper diaries, phone calls, or retrospective data collection—methods that create gaps in data quality and patient safety. Paper forms get lost, damaged, or filled in days after symptoms occur, making the data unreliable for regulatory submission. Phone call follow-ups burden coordinators and often miss critical adverse events. When safety signals are delayed, patient wellbeing is compromised and sponsors face compliance risks.
We designed our ePRO platform specifically for clinical research teams, with a focus on three priorities: patient safety through real-time alerts, high completion rates through mobile-first design, and regulatory compliance built into every workflow.
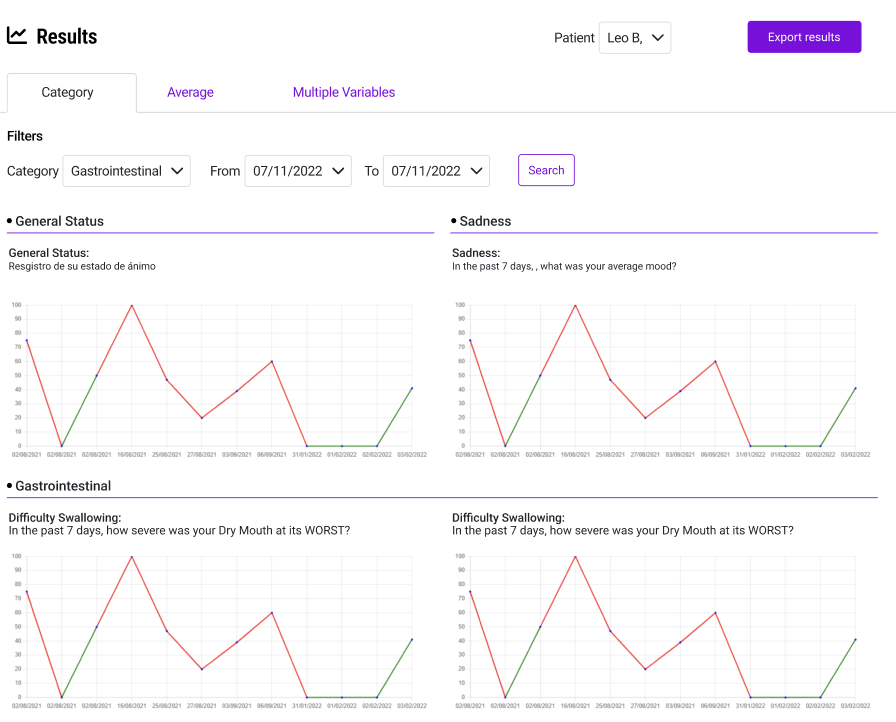
Patient safety can't wait for the next clinic visit. Our ePRO system flags adverse events the moment they're reported, sending instant notifications to clinical teams, physicians, or sponsors based on configurable severity thresholds. Every alert is logged with a complete audit trail, giving you the documentation regulators expect during inspections. When a patient reports a serious symptom at 2 AM, your team knows about it before morning rounds.
Patient safety can't wait for the next clinic visit. Our ePRO system flags adverse events the moment they're reported, sending instant notifications to clinical teams, physicians, or sponsors based on configurable severity thresholds. Every alert is logged with a complete audit trail, giving you the documentation regulators expect during inspections. When a patient reports a serious symptom at 2 AM, your team knows about it before morning rounds.

We designed for patients, not just researchers. Our surveys work on any smartphone without app downloads, load quickly on slow connections, and use simple interfaces that patients of all ages can navigate. The result: completion rates high enough that our clients stopped making follow-up calls. When patients can report symptoms in under two minutes from their couch, they actually do it—giving you the consistent, high-quality data your trial depends on.
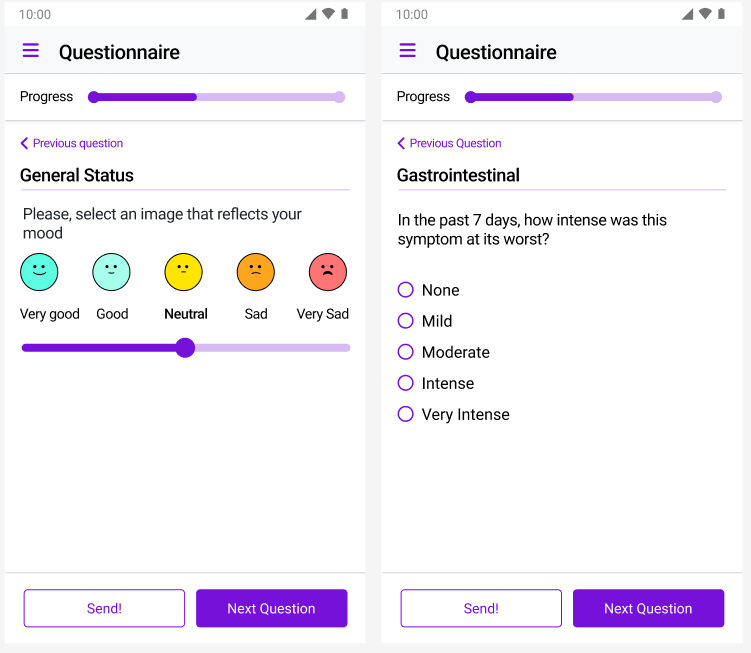
We designed for patients, not just researchers. Our surveys work on any smartphone without app downloads, load quickly on slow connections, and use simple interfaces that patients of all ages can navigate. The result: completion rates high enough that our clients stopped making follow-up calls. When patients can report symptoms in under two minutes from their couch, they actually do it—giving you the consistent, high-quality data your trial depends on.

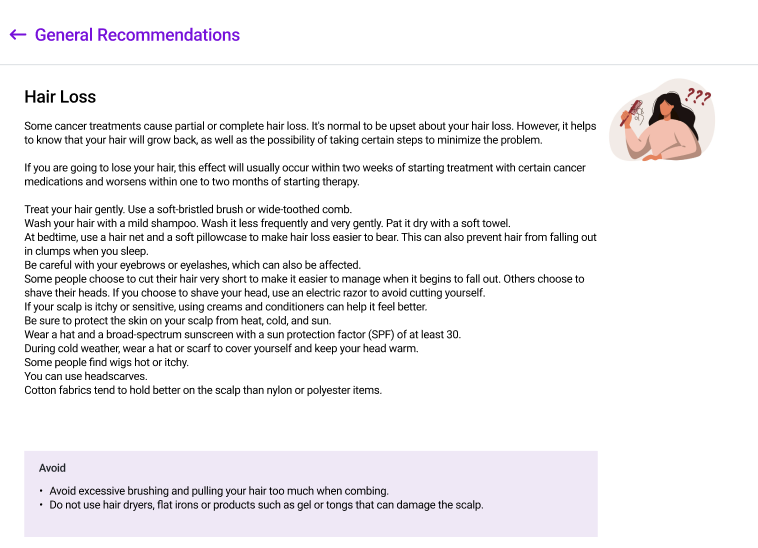
Clinical monitors juggle dozens of tasks across multiple patients and sites. Our ePRO system includes built-in task tracking and smart notifications that ensure nothing falls through the cracks. When a patient survey is due, when a response requires review, or when a follow-up window is closing—your team gets notified automatically. Every task is logged, assigned, and tracked until completion, so monitors can focus on patient care instead of spreadsheet management.
Clinical monitors juggle dozens of tasks across multiple patients and sites. Our ePRO system includes built-in task tracking and smart notifications that ensure nothing falls through the cracks. When a patient survey is due, when a response requires review, or when a follow-up window is closing—your team gets notified automatically. Every task is logged, assigned, and tracked until completion, so monitors can focus on patient care instead of spreadsheet management.

"Already deployed in clinical trials, our ePRO system has captured 2,400+ patient surveys with completion rates that eliminated the need for follow-up calls."

Let's discuss your clinical trial challenges. Schedule a consultation with our team.
